Wissenswertes über verschiedene Photoinitiatoren für die UV-Härtung
Photoinitiators for UV curing play a crucial role in the curing process. They are essential for ensuring effective UV curing. The most common types of photoinitiators for UV curing are free radical and cationic photoinitiators. Free radical photoinitiators for UV curing are the most widely used, as they perform exceptionally well in coatings, adhesives, and inks. Choosing the right photoinitiator for UV curing depends on its reaction to light, safety considerations, and the specific application requirements. The table below outlines important features:
| Art des Photoinitiators | Aushärtungsgeschwindigkeit | Kompatibilität | Safety Concerns |
|---|---|---|---|
| Typ I | Schnell | Hoch | Niedrig |
| Typ II | Langsamer | Mäßig | Potentially toxic co-initiators |
| Cationic | Variabel | Sensitive to moisture | Protonic environment affects cells |
| Naturally Derived | Variabel | Generally high | Reduced cytotoxicity |
Wichtigste Erkenntnisse
- Photoinitiators help with UV curing. There are free radical and cationic types. These are the most common kinds. Pick the type that fits your needs.
- Free radical photoinitiators work fast. They do not need extra chemicals. This makes them good for many uses.
- Cationic photoinitiators can cure when oxygen is around. They stick well to surfaces. But they can be affected by moisture.
- Naturally derived photoinitiators are safer for people. They are also better for the environment. They work well for medical and food uses.
- Always match the photoinitiator to the UV light source. This helps curing work well. It makes products strong and long-lasting.
Types of Photoinitiators for UV Curing

Photoinitiators for uv curing fit into a few main groups. These groups are based on how they start the polymerization process. The two biggest groups are free radical and ionic photoinitiators. The table below explains how these groups work:
| Kategorie | Mechanism Type | Beschreibung |
|---|---|---|
| Free Radical | Type I and Type II | Starts polymerization by making free radicals with UV light. |
| Ionic | Cationic and Anionic | Starts polymerization by making strong acids or bases. |
Free Radical Photoinitiators: Key Features
Free radical photoinitiators are used most often in uv curing. They start the reaction when they take in ultraviolet light. There are two main types: type i and type ii photoinitiators.
- Type i photoinitiators break apart when they take in light. This makes free radicals that begin the polymerization. These photoinitiators do not need other chemicals to work.
- Type ii photoinitiators need a helper called a co-initiator. When they take in light, they react with the co-initiator. This usually means taking a hydrogen atom or moving an electron. This makes free radicals that start the reaction.
The table below compares these two types:
| Typ | Mechanismus | Merkmale |
|---|---|---|
| Typ I | Cleavage-type | Takes in light, gets excited, and splits to make free radicals. |
| Typ II | Hydrogen abstraction-type | Takes in light, reacts with a co-initiator, and makes free radicals by moving electrons. |
Type i photoinitiators often work better because they do not need other chemicals. Type ii photoinitiators may not work as well if the co-initiator does not match the formula.
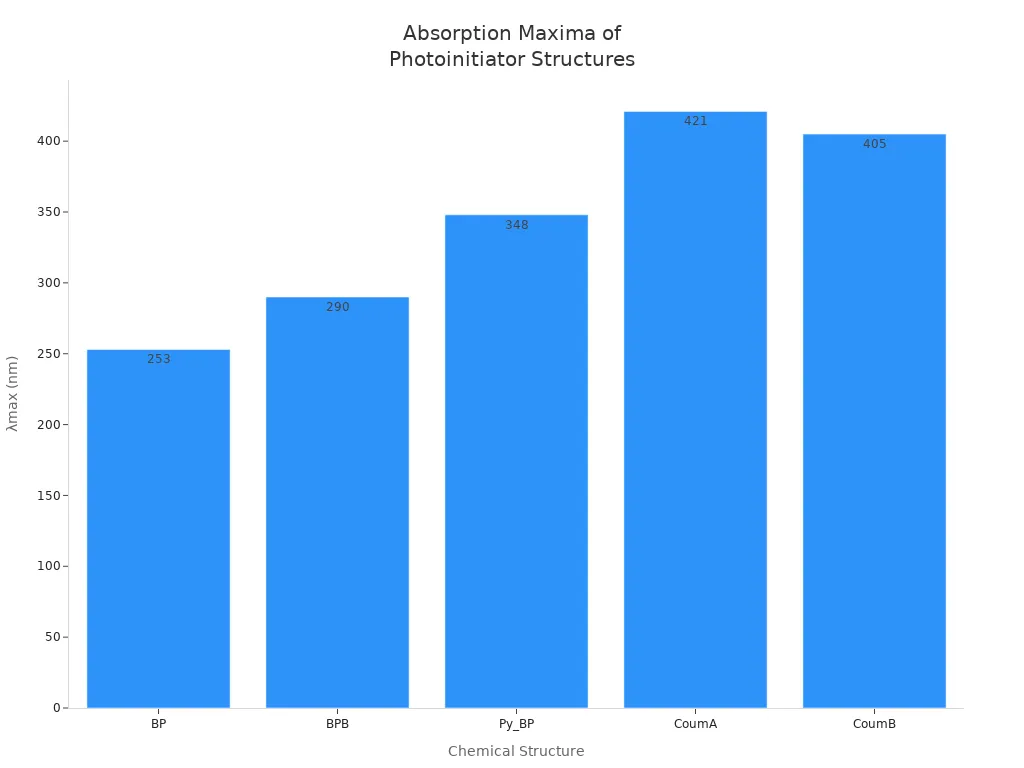
Some common chemical structures in free radical photoinitiators are BP, BPB, Py_BP, CoumA, and CoumB. These chemicals take in light at different wavelengths. The chart below shows how much light each structure can take in:
TPO is a popular free radical photoinitiator. It works well with LED lamps and cures things quickly. TPO also helps get good results in many uses.
Cationic Photoinitiators: Advantages and Drawbacks
Kationische Photoinitiatoren start the reaction by making strong acids when they get ultraviolet light. These acids then start the polymerization. Cationic photoinitiators do not have problems with oxygen stopping the reaction. This means they can cure things all the way, even in air.
Some good things about cationic photoinitiators are:
- No problems with oxygen.
- Less shrinking during curing.
- Strong sticking and toughness.
- Good resistance to chemicals.
- Can keep curing after the light is off, called “dark cure.”
But cationic photoinitiators also have some problems. They can be sensitive to water, which can slow or stop the reaction. Some types, like aryl diazonium salts, do not handle heat well and can let out gases that hurt the final product. The price and supply of materials for cationic photoinitiators can also make them hard to use in factories.
Hybrid and Naturally Derived Photoinitiators
Hybrid and naturally derived photoinitiators are new choices for uv curing. Scientists made hybrid photoinitiators by mixing things like silica with other chemicals, such as 2-chlorothioxanthone. These hybrids help spread tiny particles better and make coatings stronger.
Naturally derived photoinitiators use molecules from plants or other natural things. These photoinitiators are safer and better for the environment than man-made ones. They lower the harm to nature from photopolymerization. They also work better with living things, which is important for medical and dental uses.
Some new photoinitiators, like 1,4-benzoxazin-2-ones, help make high-quality 3D printed items. These materials are safe and work well for implants and fake body parts. Other examples are coumarin thioester photoinitiators, which take in visible light and can start both free radical and cationic reactions. These new ideas make naturally derived photoinitiators a good choice for safe and green uses.
Note: Naturally derived photoinitiators can work well with visible light, so they are good for use with new LED lamps. This helps make curing safer and saves energy in many processes.
Selection of Photoinitiators: Key Factors
Absorption and Wavelength Compatibility
A photoinitiator needs to match the UV light source. This means it must absorb the right kind of light. If the match is wrong, curing will be slow or not finish. When curing is not complete, things can stay sticky or weak.
- Photoinitiators take in certain UV wavelengths to start working.
- Free radical photoinitiators usually use light between 200 and 400 nm. They work best around 330 to 360 nm.
- Cationic photoinitiators can use both UV and visible light. Some can even use light up to 700 nm.
- Most UV lamps use light at 365, 385, 395, or 405 nm.
- In dental work, Camphorquinone is used. It works best at 468 nm.
If the system has color, pigments or fillers should not block the photoinitiator. The photoinitiator also needs to mix well in the resin. Picking the right photoinitiator means matching it to the light source.
Tip: Always check the light source and the photoinitiator’s absorption range before you start.
Curing Speed and Efficiency
Curing speed is about how fast the photoinitiator makes active particles. More photoinitiator can make things go faster. Stronger light also helps the reaction move quickly. But too much photoinitiator or light can cause problems. These problems include getting too hot or having a bad surface.
Photoinitiators for uv curing need to work well. They must make enough radicals or acids to start the reaction fast. TPO is a good choice because it works with LED lamps. It gives quick and full curing. Studies show TPO at 3% can make things very strong after 10 minutes. The amount that changes from liquid to solid is about 66% to 73% for different photoinitiators.
Good curing means the final product is strong and sticks well. The right photoinitiator makes the product better for its use.
Toxicity and Environmental Impact
Safety is very important when picking photoinitiators. Some photoinitiators can be bad for health. For example, 1-Hydroxycyclohexylphenylketone is found in many products. Studies found photoinitiators in dust, especially in nail salons. People can breathe in these chemicals, which can be risky.
| Finding | Beschreibung |
|---|---|
| Toxicity Effects | Can cause cancer, cell damage, and hormone problems |
| Occupational Exposure | Nail salon workers can get small amounts every day |
| Bioaccessibility | 10% to 42% of photoinitiators in dust can get into the body |
| Concentration Levels | Nail salon dust can have much more than normal places |
Photoinitiators can also cause allergies or hurt organs like the liver. Some are thought to maybe cause cancer in people. There are rules for materials that touch food. These rules help keep bad chemicals out of food packaging.
Picking naturally derived photoinitiators or ones with low odor and toxicity is safer. Good storage and heat stability also help stop bad byproducts from forming.
Kosten und Verfügbarkeit
Cost and supply are important for picking photoinitiators for big jobs. New tariffs in the United States have changed prices. Companies try to keep costs low and supplies steady. Prices can change fast because of supply chain problems or world events. This can make photoinitiators cost more or be hard to get.
Environmental rules can also raise costs. Companies spend money on research to make safer products. This can slow down new photoinitiators. Good suppliers and pure products help keep curing results steady. Suppliers can also help with making and using the product.
| Faktor | Beschreibung |
|---|---|
| Purity and Assay | High purity means good results and quality |
| Lieferantenzuverlässigkeit | Good suppliers give steady supply and help |
| Kosten-Wirksamkeit | Balancing price and results is important for factories |
| Application Specificity | Some photoinitiators work best with certain resins or thicknesses |
Note: Easy to make and low cost make a photoinitiator better for factories.
Picking photoinitiators means thinking about many things. Absorption, speed, safety, and cost all matter. Careful choices help get the best results for each job.
Photoinitiators for UV Curing in Applications
Industrielle Beschichtungen
Industrial coatings use photoinitiators for uv curing to make surfaces strong. These coatings protect things in cars, electronics, and packaging. TPO is good for clear coatings and electronics. It cures fast and makes things look clear. ITX is used in inks and screen printing. It can take in longer light waves. DETX works with LED-curable inks and flexible packaging. It matches new types of lights.
Engineers pick photoinitiators by looking at how well they dissolve, their color, and how fast they cure. The UV spectrum of each one helps decide if it is right for the job. Sometimes, coatings do not stick well to metals or plastics. This can cause peeling and short life. Outdoor coatings must handle sunlight and weather. They need to be stable and not break down. Factories can have trouble when making more at once. Results can change from the lab to the factory.
Tip: Try coatings on real materials first. This helps stop peeling and makes them work better.
3D-Druck
3D printing uses uv curable resins and photoinitiators to build things layer by layer. TPO is a favorite because it cures fast and works with LED lamps. Photoinitiators take in UV light and start the reaction. This turns liquid resin into solid shapes.
Some 3D printing needs materials that can mix with water. Thermal initiators help here, especially with near-infrared and visible light. Problems can be yellowing, photoinitiators moving to the surface, and toxicity. The thickness of the resin matters for printing. Good results need the right curing speed, printing speed, and monomer type.
Note: Picking the best photoinitiator helps make 3D prints sharper and stronger.
Inks and Adhesives
Inks and adhesives use photoinitiators to start reactions during uv curing. These are used in printing, packaging, and electronics. The choice depends on a few things:
| Kriterien | Beschreibung |
|---|---|
| Absorption Spectrum | Needs to match the UV lamp for good curing. |
| Reactivity and Efficiency | High reactivity makes curing fast. |
| Solubility and Compatibility | Should mix well with other parts. |
| Yellowing Tendency | Low yellowing is needed for clear uses. |
| Migration and Odor | Low migration and odor are best for food safety. |
Photoinitiators change how fast and deep curing happens. The glue’s chemistry decides how it reacts to free radicals. This affects how strong and heat-resistant it is. Cationic photoinitiators are used for special glues that need to resist chemicals.
Tip: The right photoinitiator lowers odor and migration. This makes products safer for food and medical use.
The table below lists the main good and bad points for each type:
| Photoinitiator Typ | Profis | Nachteile |
|---|---|---|
| Typ I | Makes free radicals well; used a lot | Needs strong light; does not take in all light |
| Typ II | Works with weaker light; can be used in more ways | Not as good at making free radicals; might need other parts |
People should pick photoinitiators that fit the light and curing job. They should look at how much light is taken in, how well it works, and if there are any side effects. New research is making photoinitiators safer and better for many jobs.
FAQ
Was ist ein Fotoinitiator?
A photoinitiator is a chemical that starts a reaction when it gets UV or visible light. This reaction helps change liquids into solids, like coatings or 3D prints.
How does a photoinitiator affect curing speed?
The kind of photoinitiator changes how fast curing goes. Type I photoinitiators usually work faster than Type II. Cationic types can keep curing after the light is off.
Are naturally derived photoinitiators safer?
Many naturally derived photoinitiators are less toxic. They often work better for medical or food jobs. They also help protect the environment.
Can one photoinitiator work with any UV lamp?
No, each photoinitiator takes in certain kinds of light. Users need to match the photoinitiator to the lamp’s light for the best results.