A "alma" dos materiais fotocuráveis: Fotoiniciadores
In the world of photocurable materials, there is an ingredient that, although only accounting for 2%-5% of the formula, acts like a “key” in chemical reactions, determining the success or failure of the entire curing process—it is the photoinitiator. As the core of photocuring technology, photoinitiators quickly initiate polymerization reactions after absorbing light energy, transforming liquid resins into solid materials within seconds. They are widely used in inks, coatings, adhesives, 3D printing, and other fields. The following content will systematically review its basic concepts, types, characteristics, and development trends, giving you a deeper understanding of the “great wisdom” behind this “small player.”
I.Conceptual Analysis: What are Photoinitiators?
Photoinitiators, also known as photosensitizers or photocuring agents, are substances that, under light irradiation, absorb energy at specific wavelengths, forming an excited state and subsequently generating active free radicals or cations, thereby initiating the polymerization and cross-linking reactions of monomers or oligomers.
Their mechanism of action can be summarized as follows:
They absorb photon energy in the ultraviolet (250-420 nm) or visible light (400-800 nm) region, transitioning to an excited state. Subsequently, they generate active free radicals through homolytic cleavage of chemical bonds (cleavage type) or hydrogen abstraction reactions (hydrogen abstraction type), or initiate cationic polymerization by generating strong protonic acids, ultimately promoting the curing and shaping of the system.
II. Classification of Photoinitiators
(1) Classification by Photolysis Mechanism
1.Cleavage-Type Free Radical Photoinitiators
After absorbing light energy, the molecule transitions to an excited state, and the weak bonds in its structure undergo homolytic cleavage, directly generating active free radicals.
Typical examples:
- Benzoin and its derivatives (e.g., benzoin ethers)
- α-hydroxyketone derivatives (e.g., 1173, 184, 2959)
- α-aminoalkylacetophenones (e.g., 907, 369)
- Acylphosphine oxides (e.g., TPO, 819)
2.Hydrogen Abstraction-Type Free Radical Photoinitiators
The excited state photoinitiator abstracts a hydrogen atom from a hydrogen atom donor (such as a monomer or prepolymer), making it an active free radical.
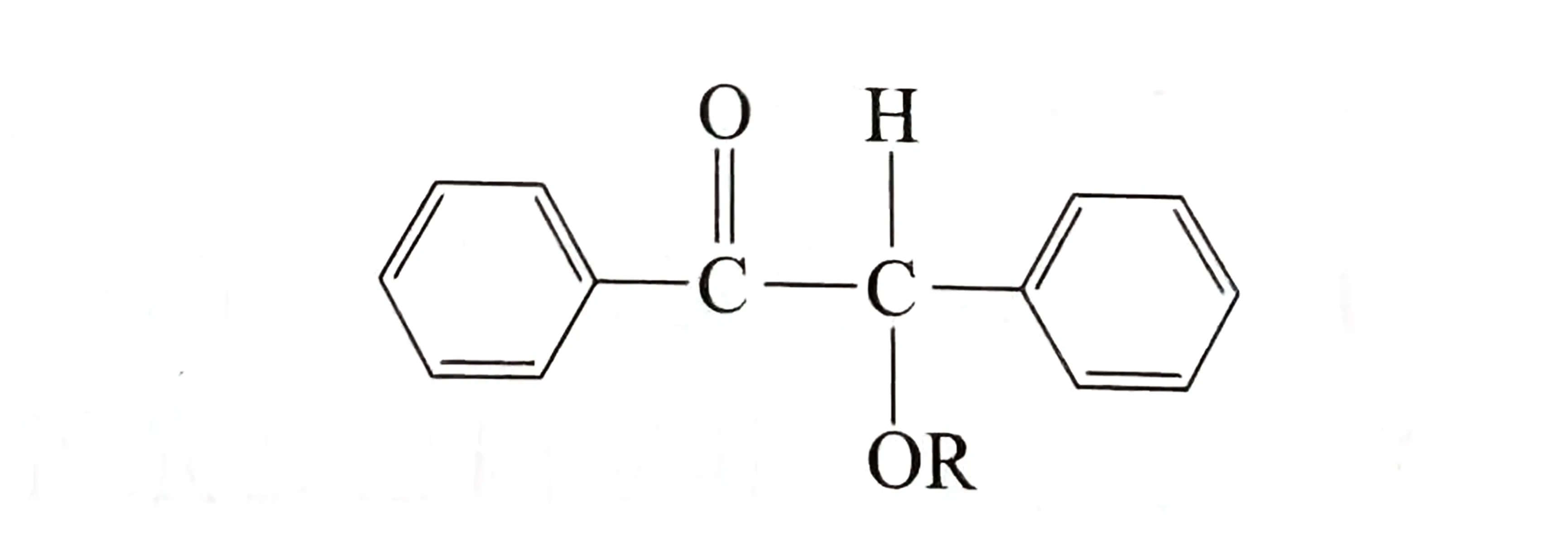
Typical examples:
- Benzofenona e seus derivados
- Thioxanthone derivatives (e.g., ITX, DETX)
- Anthraquinone derivatives (e.g., 2-ethylanthraquinone)
-
Fotoiniciadores catiônicos
After irradiation, they produce superstrong protonic acids (Brønsted acids or Lewis acids), initiating cationic polymerization of monomers such as epoxides and vinyl ethers.
Typical examples:
- Iodonium salts, sulfonium salts, iron arene salts, etc.
- Characteristics: Small curing shrinkage, strong adhesion, and not inhibited by oxygen.
(2)Classification based on structural characteristics
| Categoria | Typical Examples | Absorption Wavelength(nm) | Characteristics and Applications |
| Benzoin derivatives | Benzoin, benzoin ether | 300~400 | It is inexpensive, but has poor thermal stability and is prone to yellowing, and has therefore been gradually phased out |
| Benzoin derivatives | BDK (α,α’-dimethylbenzoin acetal) | 254, 337- 390 | It exhibits high reactivity but is prone to yellowing (the photodegradation products contain quinone-like structures) |
| Acetophenone derivatives | DEAP | 242, 325 | It offers high efficiency but has poor thermal stability and is relatively expensive |
| α-hydroxy ketones | 1173, 184, 2959 | 245~333 | It has good thermal stability and resistance to yellowing, and is widely used in topcoats, wood coatings, etc |
| α-aminoketones | 907, 369 | 230~324 | High activity, suitable for colored systems; some varieties have limitations due to yellowing or toxicity |
| Thioxanthones | ITX, DETX | 257~430 | Long-wavelength absorption, commonly used for curing thick layers or colored systems |
| Anthraquinones | 2-ethylanthraquinone | 256~430 | It is insensitive to oxygen inhibition and is often used in solder mask inks. |
III. Characteristics and Selection Principles of Photoinitiators
(1) Selection Principles
- Spectral Matching:The absorption spectrum of the initiator should overlap with the emission spectrum of the light source, and have a high molar extinction coefficient.
- High Efficiency and Economy: Good solubility, high reactivity, and low dosage.
- Estabilidade:Stable during storage and does not decompose below 85°C.
- System Compatibility: Select an initiator with appropriate activity based on the type of prepolymer/monomer.
- Combined Use: Using multiple initiators in combination to broaden the absorption wavelength range and improve curing speed and depth.
- Environmental Friendliness and Safety: Low odor, low toxicity, and environmentally friendly.
- Controllable Cost: Simple synthesis process and readily available raw materials.
(2) New Development Trends
- Hybrid Systems:Combining free radical and cationic photoinitiators, offering the advantages of rapid curing, low shrinkage, and high adhesion.
- Visible Light Photoinitiators:Such as titanocene compounds (Irgacure 784), with absorption wavelengths extending to 500 nm, suitable for visible light curing systems.
- Water-based Photoinitiators: Introducing hydrophilic groups (such as sulfonates) to improve water compatibility, suitable for environmentally friendly water-based coatings.
- Macromolecular Photoinitiators: Incorporating photoinitiators into polymer chains to improve compatibility and reduce migration and odor.
- Dual Curing Systems:Combining photocuring with thermal curing, moisture curing, etc., to solve the problem of curing in shaded areas and improve material performance.
Conclusion: Small Components Drive a Big Future
Although photoinitiators constitute only a small percentage of the formulation, they are crucial for achieving efficient and precise curing of photocurable materials. With increasing environmental demands and expanding application scenarios, photoinitiators are continuously evolving towards higher efficiency, greater safety, broader applicability, and improved environmental friendliness. From ultraviolet to visible light, from oil-based to water-based systems, and from single-trigger to dual-curing mechanisms, every breakthrough in this technology is injecting new vitality into fields such as green manufacturing, intelligent coatings, and 3D printing. In the future, photoinitiators will continue to play a pivotal role in driving photocurable technology towards even broader applications.