Apa yang Membuat BZA Monomer CAS 2495-35-4 Unik dalam Struktur dan Sifat Kimia
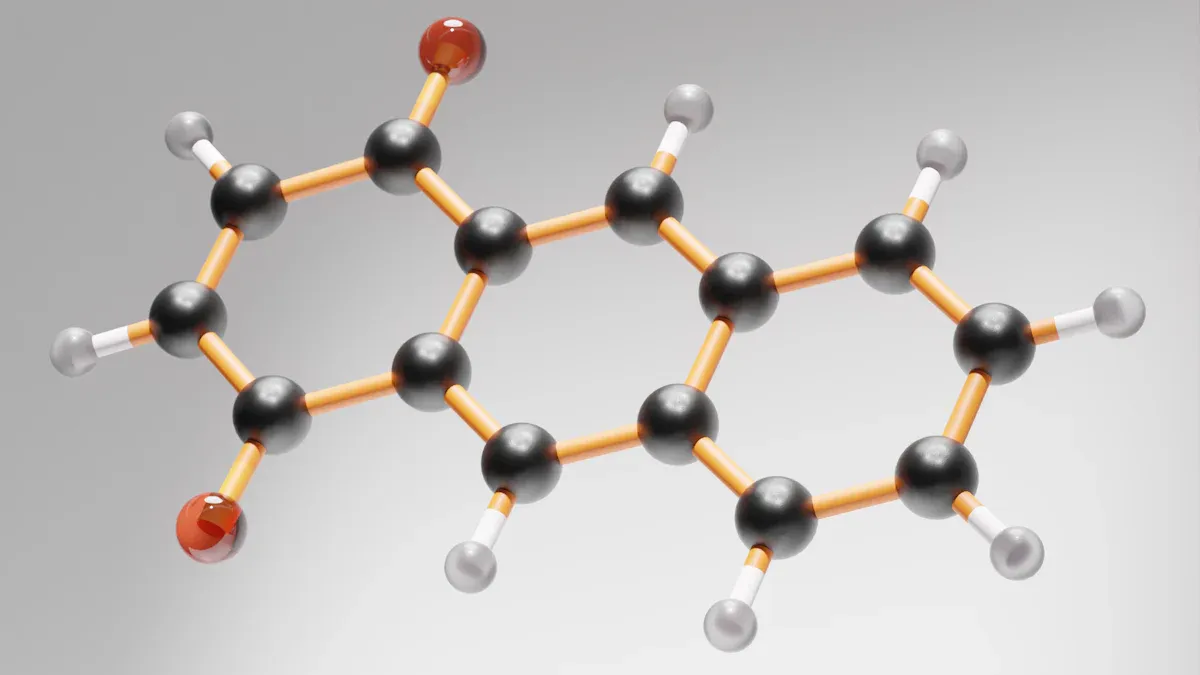
BZA monomer cas 2495-35-4 is special because of its unique structure. This monomer has an acrylate group and a benzyl ring. These parts give it better heat stability and stronger strength. It also has a high refractive index. Many chemists and material scientists like cas number 2495-35-4 for its low viscosity. They also value its special optical properties. Knowing these qualities helps experts connect structure to function in new materials.
Hal-hal Penting yang Dapat Dipetik
- BZA monomer CAS 2495-35-4 has a special structure. It has a benzyl ring and an acrylate group. This helps it stay strong and stable when heated.
- BZA monomer has a high refractive index. This makes it great for coatings and adhesives. It gives them clear looks and helps them last longer.
- When BZA monomer is used in polymerization, it makes strong and clear materials. This helps industries like electronics and coatings.
- You should always store BZA monomer with a stabilizer like MEHQ. This stops bad reactions and keeps its good features.
- BZA monomer’s special parts help make new materials. This makes it a smart pick for material scientists.
BZA Monomer CAS 2495-35-4 Structure
Molecular Formula and Structure
Benzyl acrylate has a simple structure that is important. Chemists call its formula C10H10O2. This means each molecule has ten carbon atoms, ten hydrogen atoms, and two oxygen atoms. Its molecular weight is 162.19 grams for every mole. The table below shows these facts clearly:
| Properti | Nilai |
|---|---|
| Rumus Molekul | C10H10O2 |
| Berat Molekul | 162.19 g/mol |
Benzyl acrylate has two main parts in its structure. One part is the benzyl group. The other part is the acrylate group. These two groups join together to make the whole molecule. The benzyl group comes from benzene, which is a ring with six carbon atoms. The acrylate group has a double bond between two carbon atoms and a carboxyl group. This double bond makes benzyl acrylate react easily.
Benzyl and Acrylate Groups
The benzyl group gives benzyl acrylate special features. The ring shape of benzyl makes it strong and stable. This helps benzyl acrylate stand up to heat and light. The acrylate group lets the molecule work well in polymer reactions. When scientists make a polymer, the acrylate group helps connect many benzyl acrylate molecules.
Benzyl acrylate is special because it uses the best parts of both groups. The benzyl group makes the refractive index higher. The acrylate group helps it react quickly. This means benzyl acrylate can make materials that are strong and clear. Many industries use benzyl acrylate for coatings, adhesives, and other things. Its unique structure makes it a great choice for new polymer designs.
Note: The mix of benzyl and acrylate groups gives benzyl acrylate its special features. This is why scientists look at benzyl acrylate when they want to make new materials.
Unique Properties of Benzyl Acrylate
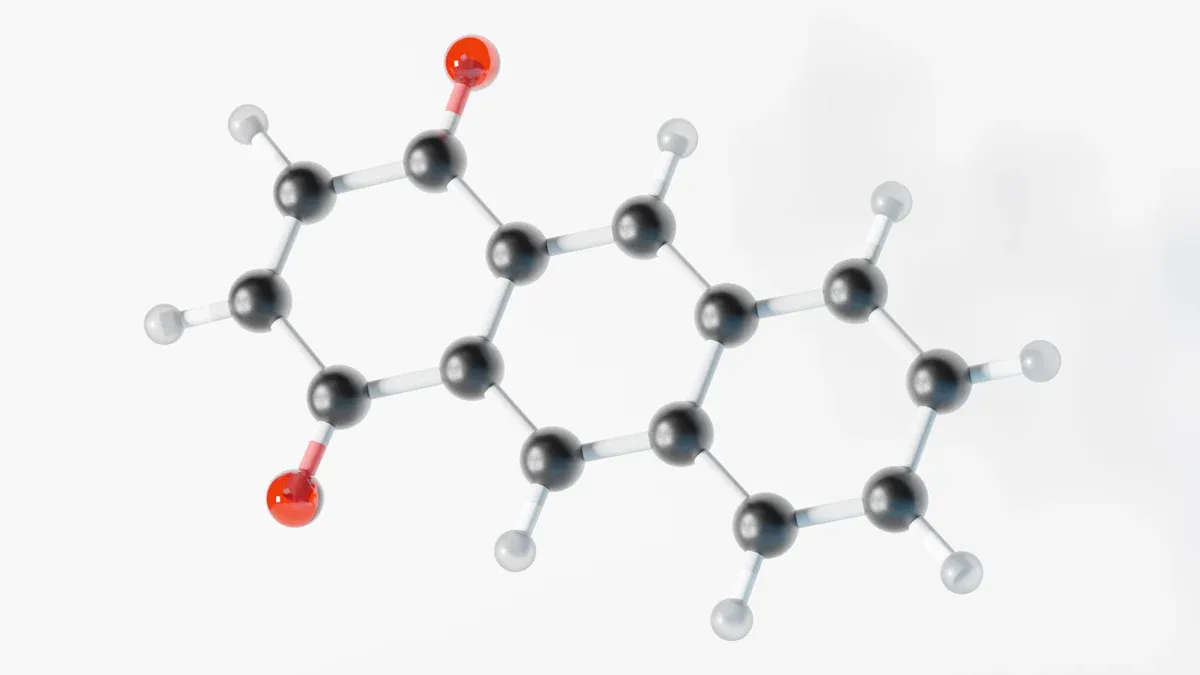
Polymerization and Reactivity
Benzyl acrylate is good at joining in polymerization. The acrylate group reacts fast with other monomers. This helps make long chains called polymers. Scientists use benzyl acrylate to create materials with special features. The benzyl group gives extra stability during these reactions.
Researchers have checked how benzyl acrylate acts in different settings. The table below shows a study about poly(benzyl acrylate) in supercritical carbon dioxide. This research helps experts learn how the polymer forms and what changes its properties.
| Judul Penelitian | Deskripsi |
|---|---|
| Cosolvent effect on the phase behavior for the poly(benzyl acrylate) and poly(benzyl methacrylate) in supercritical carbon dioxide | This study shares cloud-point data for poly(benzyl acrylate) and poly(benzyl methacrylate) in supercritical carbon dioxide. It shows how cosolvents and different conditions affect phase behavior. |
The benzyl group also helps the polymer stand up to heat and light. This means products made from benzyl acrylate last longer. They keep their features better than other acrylate monomers.
Volatility and Stability
Benzyl acrylate does not evaporate quickly like many other acrylate monomers. The benzyl ring lowers volatility and makes the compound more stable. When stored, benzyl acrylate needs protection from unwanted polymerization. If left alone, it can start to react and form a polymer by itself.
To stop this, manufacturers add a stabilizer called MEHQ. The table below shows how much MEHQ is best for benzyl acrylate.
| Stabilizer | Recommended Concentration (ppm) |
|---|---|
| MEHQ | 200 – 400 |
This stabilizer keeps benzyl acrylate safe when stored and moved. The benzyl group also helps the acrylate resist breaking down from light or heat. These features make benzyl acrylate good for making strong and lasting materials.
Tip: Always store benzyl acrylate with a stabilizer. This keeps its features safe and stops early reactions.
Refractive Index and Hydrogen Bonding
Benzyl acrylate bends light more than many other acrylate monomers. The benzyl group makes this effect stronger. Because of this, benzyl acrylate works well in products that need special optical features, like clear coatings and advanced adhesives.
- Benzyl acrylate is known for its high refractive index.
The molecule also has places that can accept hydrogen bonds. These spots help benzyl acrylate mix well with other chemicals. This improves optical features and makes the polymer useful in many ways.
When compared to other acrylate monomers, benzyl acrylate has a special mix of features. The benzyl group gives extra stability and a higher refractive index. The acrylate group allows fast and easy polymerization. These features make benzyl acrylate a top pick for scientists who want strong and clear materials.
Applications of BZA Monomer
Advanced Polymer Synthesis
Scientists use bza monomer cas 2495-35-4 to make new polymers. The monomer has a reactive acrylate group and a benzyl ring. These parts let scientists try different ways to make polymers. One common way is called free radical polymerization. Chemists use peroxides or UV light to start this process. This makes polybenzyl acrylate and other copolymers. These polymers have special features like a high refractive index and better stability. Making these materials helps create optical films and other strong products.
- Free radical polymerization with bza monomer cas 2495-35-4 makes polymers with better optical features.
- The materials can be made into thin and light films.
- These films control how light passes through and bounces off them.
Specialty Coatings and Adhesives
Bza monomer cas 2495-35-4 is important for special coatings and adhesives. Its structure helps things stick better, bend more, and last longer. The benzyl group changes how the polymer acts in heat and helps it stick to many surfaces. The acrylate group makes the polymer form quickly and well. In coatings, this monomer makes them shinier and stronger against weather. It also makes coatings tougher so they last longer. Many companies use these coatings in hard conditions. In adhesives, the monomer lets people pick how strong the glue should be. This helps make bright inks and helps colors mix well. Pure benzyl acrylate gives steady results in these uses.
- Special coatings with bza monomer cas 2495-35-4 stand up to bad weather.
- Adhesives made with this monomer are strong and bendy.
- The monomer helps inks mix colors well.
Material Science Uses
Material scientists like bza monomer cas 2495-35-4 because it can make many kinds of materials. The polymer’s special features, like a high refractive index and good stability, help in making optical parts. These parts include prisms, diffusers, and covers for electronics. The monomer is also used to make optical films. Its structure lets scientists design materials that change how light and color work. Researchers use it to make new plastics and special mixes. The many materials made from this monomer help new ideas in many areas.
Note: The mix of a benzyl ring and acrylate group in bza monomer cas 2495-35-4 gives it features that help coatings, adhesives, inks, and advanced polymers. This makes it a smart pick for new material science.
BZA monomer CAS 2495-35-4 is different because of its special structure. It has a benzyl ring and an acrylate group. These parts make it flow easily and bend light well. This helps people make strong coatings and clear acrylic resins. Many products use BZA to work better. Scientists use this information to pick good materials for new projects. Learning more about BZA could help make better and more useful materials later on.
PERTANYAAN YANG SERING DIAJUKAN
What is the main chemical feature of BZA monomer CAS 2495-35-4?
BZA monomer has two important parts. It has a benzyl ring and an acrylate group. These parts make it stable and help it react fast. Its special structure makes it different from other acrylate monomers.
How does BZA monomer improve polymer properties?
BZA monomer helps polymers handle heat better. It also makes them clearer. The benzyl ring raises the refractive index. The acrylate group lets it join with other molecules quickly. These things help make strong and see-through materials.
Is BZA monomer safe to handle?
You should always use gloves and goggles with BZA monomer. It can bother your skin and eyes. Keep it with a stabilizer like MEHQ so it does not react by itself.
Where do scientists use BZA monomer?
Scientists use BZA monomer in special coatings and adhesives. It is also used in optical films. The monomer helps make things for electronics, inks, and new plastics.
