Factors Affecting Pigment Color
Numerous factors influence pigment color, among which chemical structure, crystal form, and surface treatment are decisive factors affecting both color and secondary properties. The color of a pigment is primarily determined by its chemical structure. However, the type of crystal form, shape, particle size, and surface treatment can also alter its color. For example, among phthalocyanine blues, the α crystal form exhibits a red hue, the β crystal form displays a green hue, and the ε crystal form produces a phthalocyanine blue pigment with a redder hue than the α form. Secondary properties of pigments—such as crystal stability, lightfastness and weather resistance, hiding power and transparency, tinting strength, gloss, rheology, dispersibility, heat resistance, and chemical resistance—are increasingly significant in market differentiation and segmentation.
Chemical Structure
Based on general chemical structure, pigments can be categorized into inorganic pigments, organic pigments, and effect pigments.
1) Inorganic Pigments:Classified by chemical structure, these include titanium dioxide, carbon black, iron oxide series, lead chromate series, chromium series and cadmium series oxides, ultramarine blue, bismuth vanadate, and composite inorganic pigments.
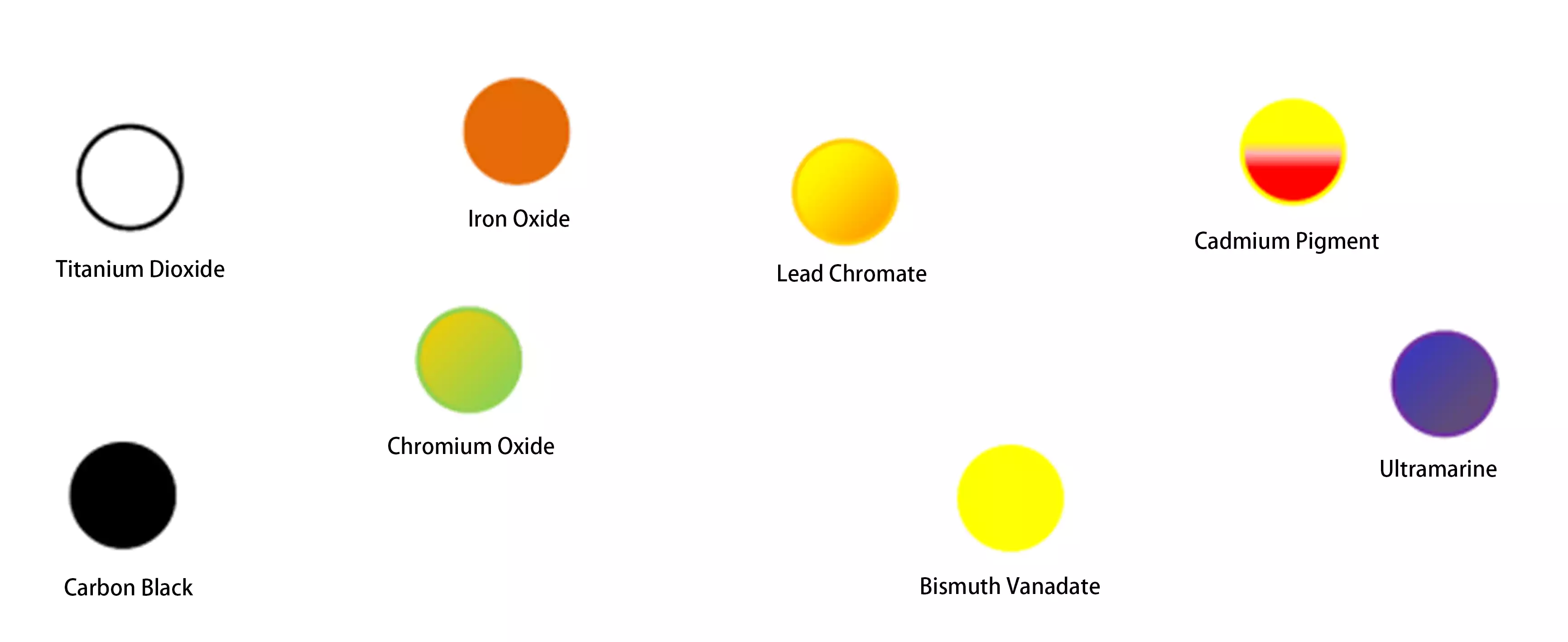
2) Organic Pigments: Organic pigments are broadly categorized by chemical structure into azo pigments, polycyclic pigments, and metal complex pigments. Azo pigments are predominantly traditional organic pigments with poor colorfastness, though exceptions exist such as benzimidazolone. Diazo condensation pigments, however, belong to the category of high-performance organic pigments. Polycyclic and metal complex pigments represent high-performance organic pigments with excellent color fastness and resistance properties.
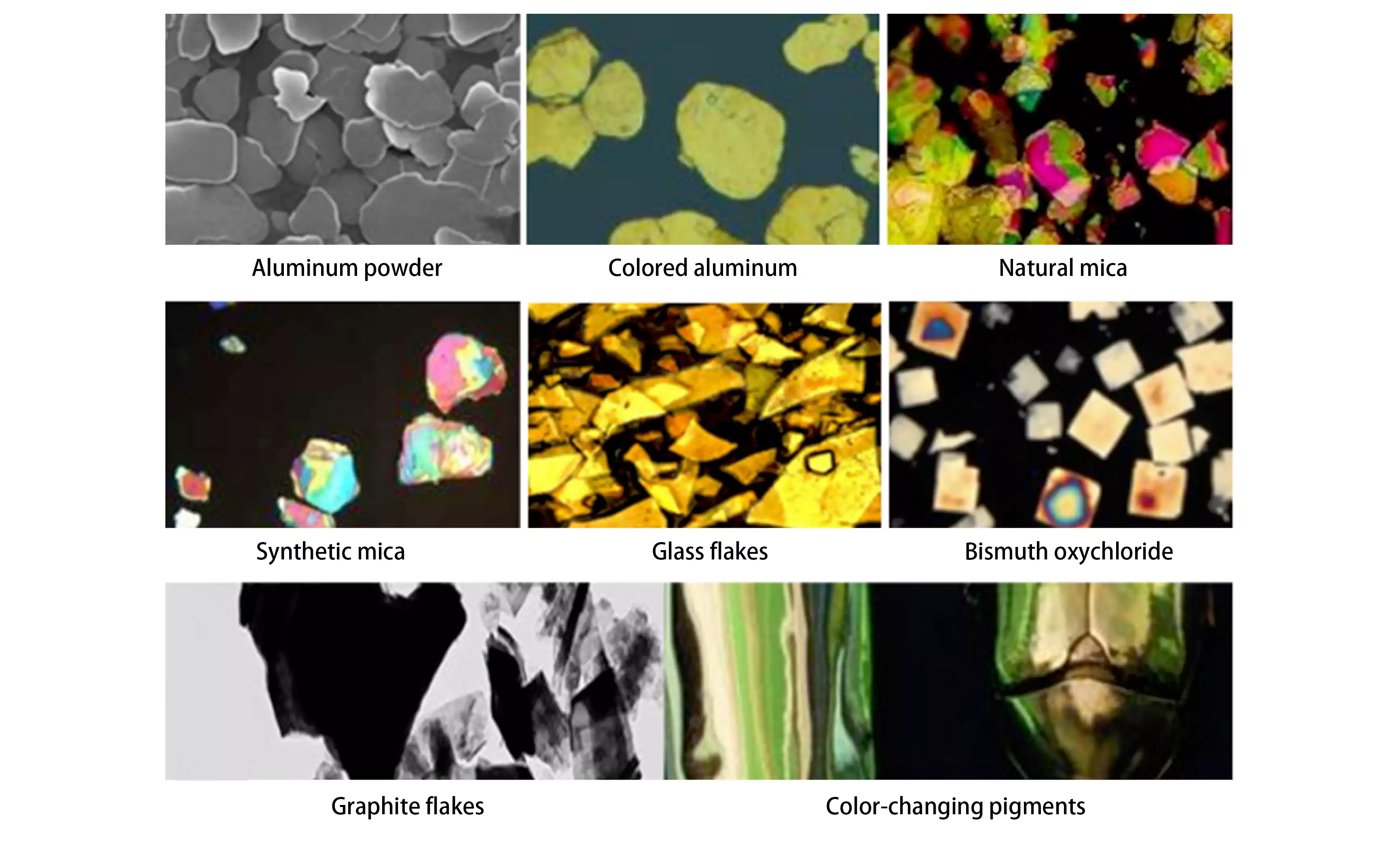
3) Effect Pigments: Aluminum powder, copper powder, metallic aluminum, natural mica, synthetic mica, glass flakes, bismuth oxychloride, graphite flakes, polymer structural colors, and color-changing pigments, among others.
Crystal and Surface Treatment
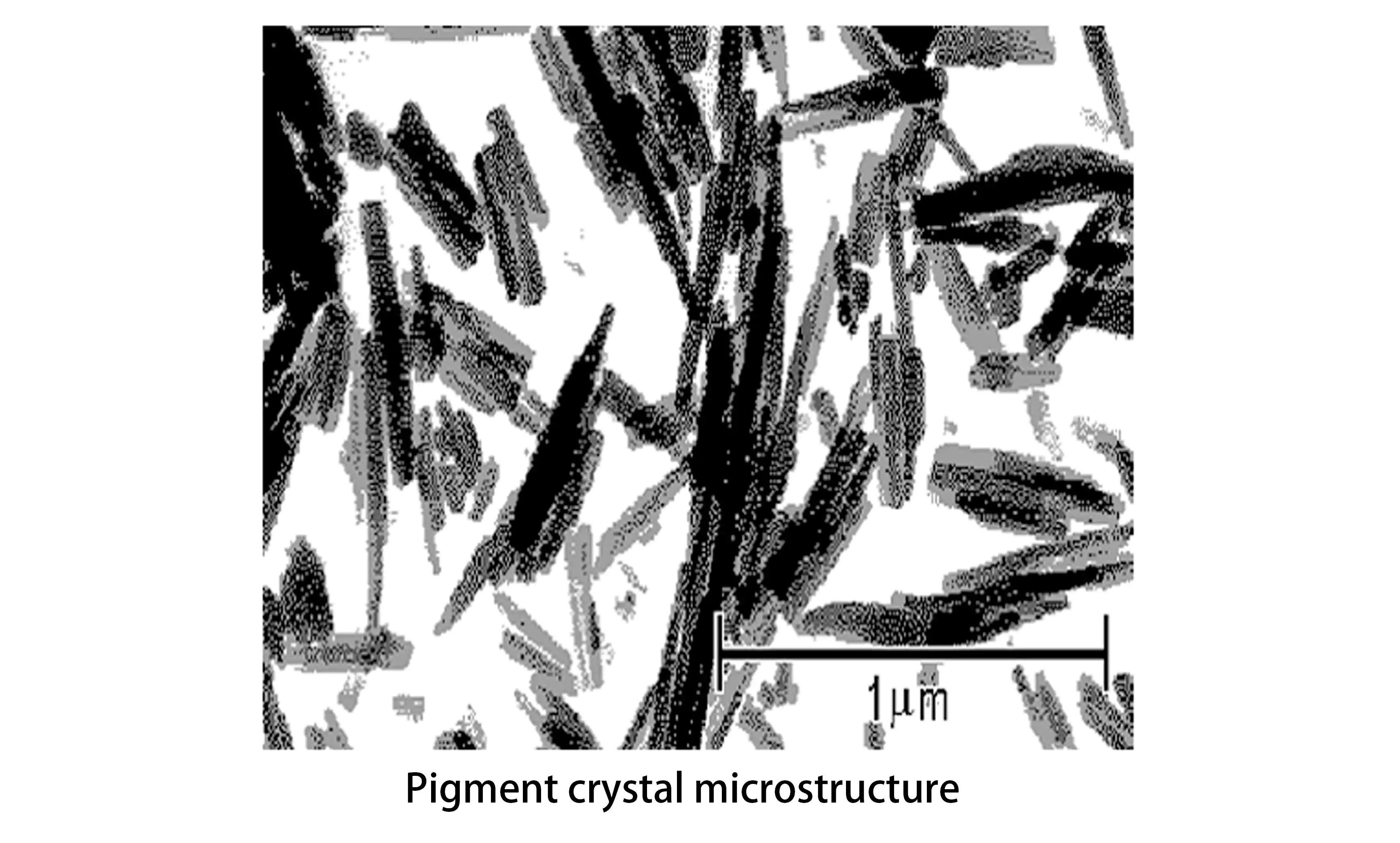
The particle size, size distribution, crystal shape, crystal structure, and crystallinity of pigment crystals all influence color performance and secondary properties. Powders exhibit either crystalline or amorphous structures, while pigments always exist in crystalline form. Pigment crystals represent the smallest particles of pigment, consisting of atoms or molecules arranged and assembled in specific patterns. Their distinct shapes and microstructures can be observed using electron microscopy.
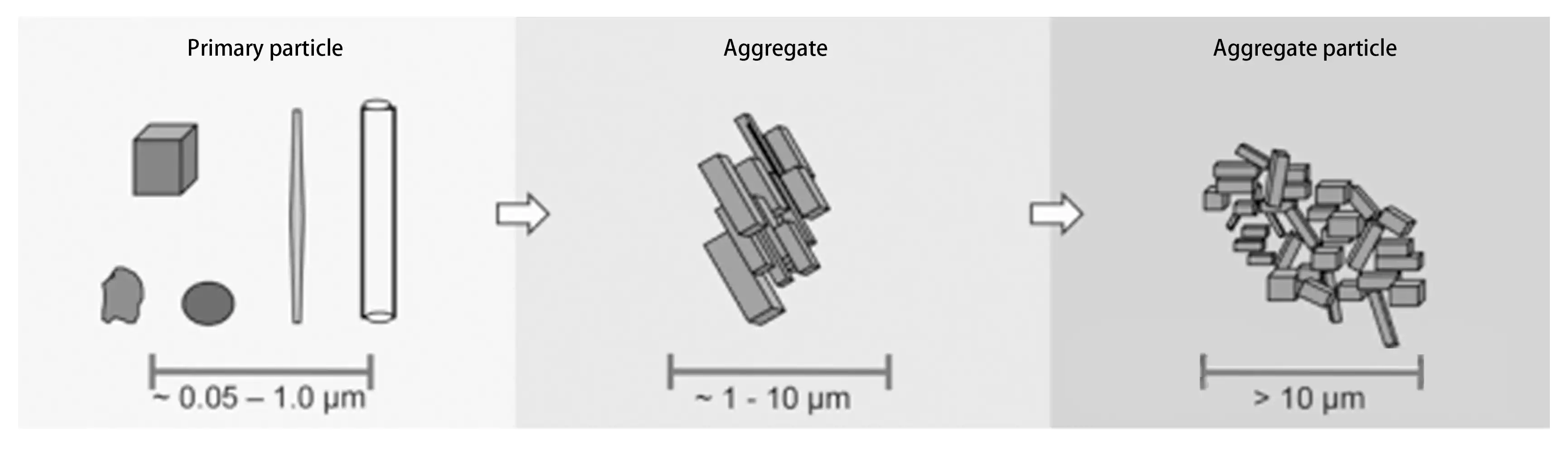
 The smallest particles present in pigment dispersions are called primary particles, with diameters ranging from approximately 0.05 to 1 μm. They exhibit various shapes such as cubes, spheres, rods, and needles.
The smallest particles present in pigment dispersions are called primary particles, with diameters ranging from approximately 0.05 to 1 μm. They exhibit various shapes such as cubes, spheres, rods, and needles.
Due to their high surface energy, primary particles exhibit a strong tendency to spontaneously aggregate and reduce surface energy. They typically form tightly structured aggregates through random edge-to-edge or face-to-face bonding. These aggregates have larger diameters than primary particles, generally ranging from 1 to 10 μm. During filtration and drying processes in pigment manufacturing, primary particles and aggregates, or aggregates themselves, agglomerate to form larger, more loosely structured agglomerates with particle sizes exceeding 10μm.
Pigment crystal characteristics are typically manifested through the following properties, each exerting varying degrees of influence on pigment performance. Particle size, shape, and size distribution exert particularly significant effects on pigment properties.
- Particle Size Generally small, measured in micrometers (μm).
- Particle size distribution, commonly measured by parameters like D10, D50, and D90. For example, a D50 of 20μm indicates that 50% of the pigment particles have an average diameter of 20μm.
- Crystal particle shape, such as acicular, spherical, cubic, or other forms.
- Crystal structure: A single pigment may exhibit multiple crystal forms. For instance, phthalocyanine blue has α, β, and ε forms, while titanium dioxide exists as anatase, rutile, and brookite. Different crystal structures yield varying color properties and secondary characteristics.
- Crystallinity primarily affects color fastness. Increasing crystallinity enhances a pigment’s lightfastness, weather resistance, and heat resistance while also improving its rheological properties, solvent resistance, and resistance to color bleeding.
Surface treatment is a critical process and technology in pigment processing. The primary function of surface treatment is to alter particle surface polarity, ensuring good compatibility between the pigment and the application medium. This improves application properties such as dispersion, rheology, resistance, and stability of the dispersion system. The same pigment can be processed through different surface treatments to produce product types with specific application properties, such as easy dispersion, anti-flocculation, high hiding power, or high transparency, as well as suitability for solvent-based or water-based applications.
The Effect of Particle Size on Pigments
The particle sizes of pigments with different structures also vary greatly. Generally, inorganic pigments have larger particle sizes than organic pigments. Among inorganic pigments, carbon black has very small particle sizes, even smaller than some organic pigments, while titanium dioxide and iron oxides have larger particle sizes. The particle size of iron oxide red can reach dozens of times that of titanium dioxide.
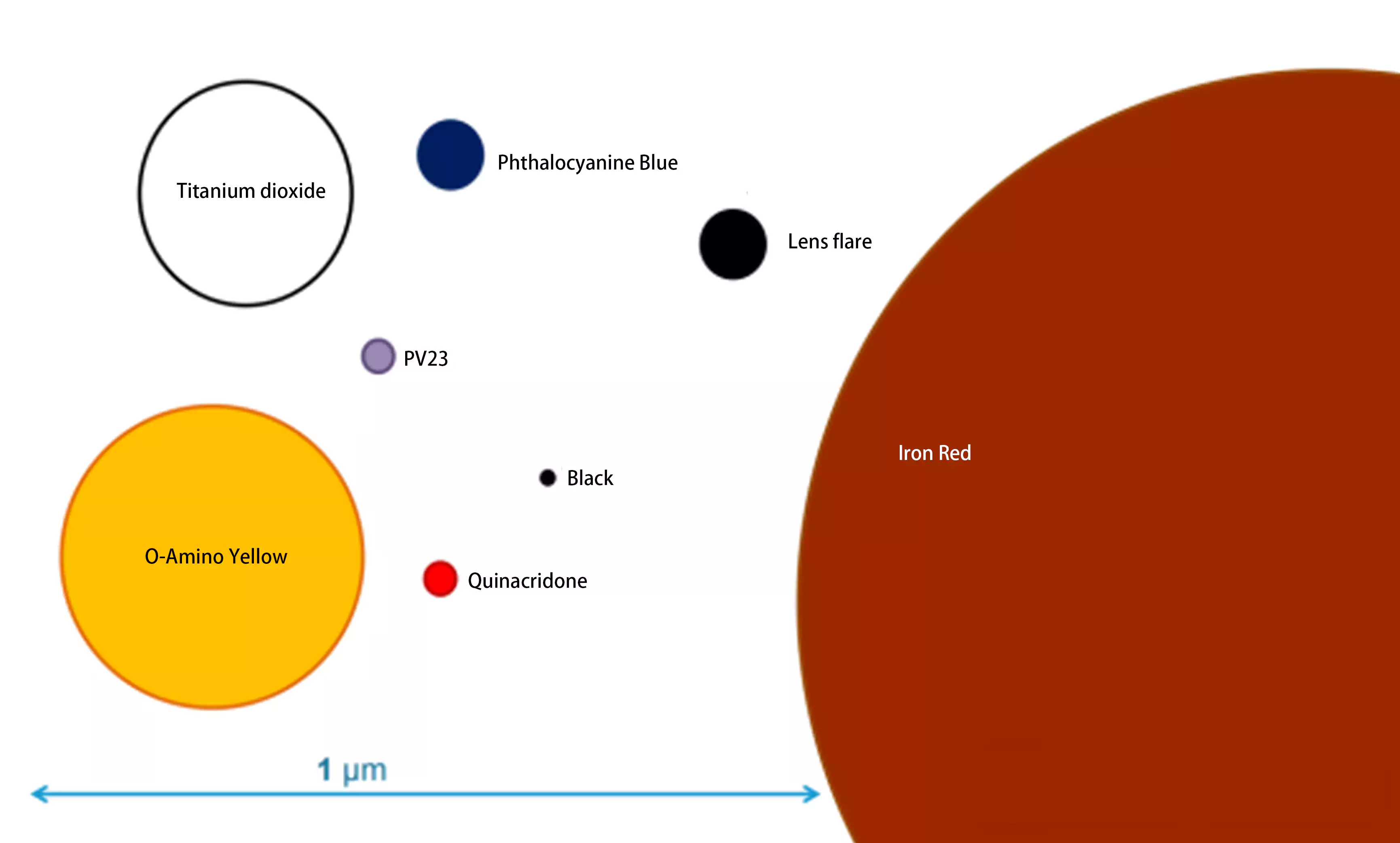 Effect of Particle Size on Hue
Effect of Particle Size on Hue
The hue of pigments with identical chemical structures varies with particle size. For a given pigment, the color angle increases as particle size decreases and decreases as particle size increases. When the particle size of a specific pigment decreases, its hue shifts counterclockwise on the color wheel. Conversely, when particle size increases, the hue shifts toward adjacent hues in a counterclockwise direction.
Effect of Particle Size on Surface Area and Properties
The smaller the pigment particle size, the larger its specific surface area. Smaller particles exhibit stronger adsorption capacity and higher viscosity in the medium, naturally requiring greater quantities of dispersants for dispersion and viscosity reduction. Different particle sizes of the same pigment exhibit distinct characteristics in color saturation, rheology, transparency or hiding power, tinting strength, and weather resistance.
|
Small particle size |
Performance | Large particle size |
| High transparency | Transparency/Opacity |
High coverage |
| High | Tinting Strength |
Low |
|
Poor |
Rheology |
Good |
| Poor | Weather Resistance |
Good |
| High | Color Saturation |
Low |
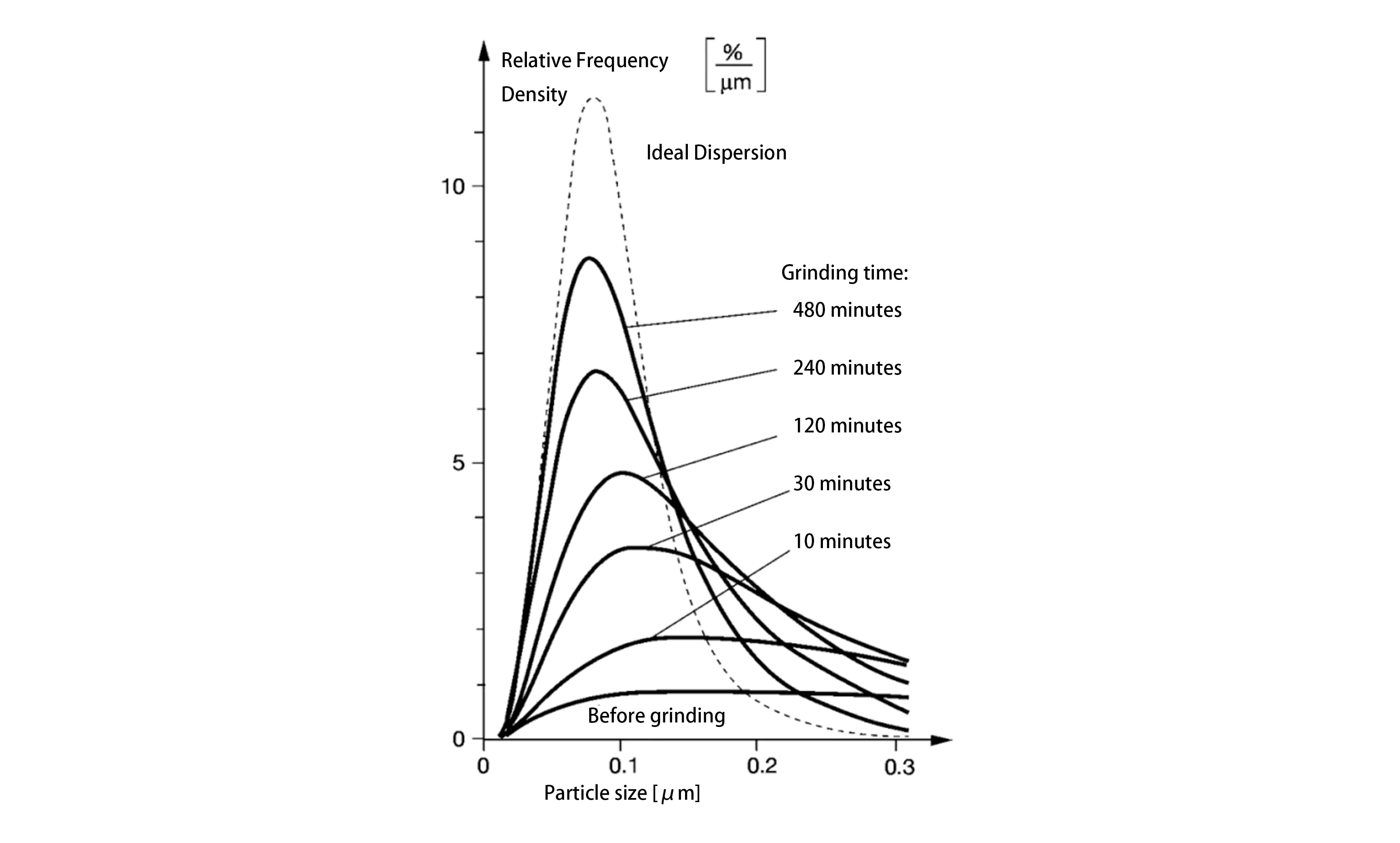
The Effect of Particle Size Distribution on Grinding and Dispersion
The grinding and dispersion of pigments involves using shear forces to break agglomerates into aggregates, or ideally into primary particles, thereby enhancing performance. Simultaneously, pigments with narrower particle size distributions exhibit higher color saturation and tinting strength. The particle size distribution diagram below, showing β-phase phthalocyanine blue in an aqueous system after varying dispersion times, clearly demonstrates that increasing grinding time results in progressively finer average particle sizes and a more concentrated distribution, approaching an ideal distribution. However, excessive grinding can sometimes lead to undesirable effects such as hue shifts and diminished performance.
 In addition to the above factors, the shape of the crystal also significantly influences the rheological properties of pigments. Aggregates formed by pigment particles with plate-like or rod-shaped crystals exhibit larger volumes compared to aggregates from particles with other crystal shapes. Under identical conditions, these aggregates possess lower viscosity and demonstrate relatively superior dispersibility within the dispersion medium.
In addition to the above factors, the shape of the crystal also significantly influences the rheological properties of pigments. Aggregates formed by pigment particles with plate-like or rod-shaped crystals exhibit larger volumes compared to aggregates from particles with other crystal shapes. Under identical conditions, these aggregates possess lower viscosity and demonstrate relatively superior dispersibility within the dispersion medium.
